The management of most oil wells at some point will involve EOR methods. Polymer floods and alkaline surfactant polymer (ASP) floods make up more than 50% of tertiary recovery globally. While polymer-based recovery (with or without other additives) typically exhibits an excellent financial profile, once used, they inhibit the ability to treat water for injection or disposal. This inability to properly process water has discouraged further adoption of polymers for tertiary recovery due to the potential that exists for formation damage or reduced well productivity.
Produced water intended for discharge or upcycling requires substantial treatment to meet quality requirements. The methods currently applied to remove oil and solids from produced water have not substantively changed in decades. They include cyclonic technologies (hydrocyclones), induced or dissolved gas, media filtration (sand, crushed glass, activated carbon, nutshells) and sometimes polishing steps like absorbent media.
The performance of all of these technologies is significantly challenged by the water viscosity and anionic charge that result from the introduction of polymers, as shown in Figure 1.
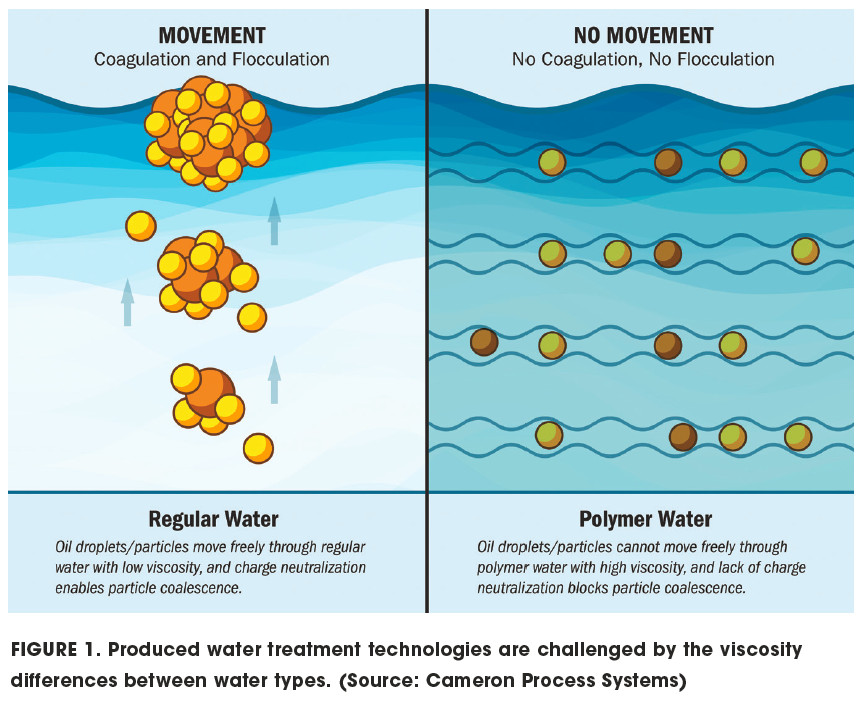
The polymers commonly used in polymer and ASP floods are high molecular-weight linear anionic homopolymers or copolymers. They include hydrolyzed polyacrylamide or acrylamide copolymers such as acrylamide/acrylic acid. Coagulation—the neutralization of the oil droplet/particle surface charges—is generally affected by the addition of a cationic material such as iron or aluminum salts. The oil droplets/particles exhibit like negative charges, preventing them from colliding and combining to become larger; separation velocity increases with the square of the particle size. Particles collide and combine to form larger particles when cationic coagulants neutralize these negative surface charges.
When the anionic polymers are used in polymer and ASP floods, the resulting polymer water requiring treatment will have an anionic charge so strong that it cannot be easily neutralized by the addition of a coagulant.
To neutralize the surface charges of the droplets/particles, the majority of charge sites on the polymer molecules must be neutralized. This can require concentrations of cationic material so high that it would far exceed the viability of most opex budgets. For these reasons, most operators are reluctant to embrace coagulation as a water treatment strategy in polymer flood or ASP waters.
The process pathways available to resolve the technical challenges associated with polymer and ASPs are represented in Figure 2. The focus has been on adjusting/altering the flow of oil through water (continuous phase). But there are other approaches that involve targeting the oil particles/droplets (dispersed phase) and the physical chemistry of the polymers used. Investing in these approaches could yield a polymer flood formula that would enhance reservoir recovery while eliminating water treatment roadblocks.

Focus on the continuous phase
The impairment of the performance of hydrocyclones, flotation cells and media filters is, in large part, attributable to the reduction of the ability to affect coagulation and the increased drag on oil particles/droplets caused by the elevated viscosity.
Initial efforts to improve treatability targeted the destruction of the polymer to decrease solution viscosity, which would reduce drag. If the polymer chain is broken, the viscosity will be reduced, minimizing some of the negative effects on water processing associated with viscosity. Produced water returning to the surface contains only a residual amount of functional polymer and typically has a fluid viscosity that has been reduced to ≤ 2 centipoise because much of the polymer has been broken into pieces by the formation.
Shearing the polymer will reduce the water treatment challenges associated with viscosity but not those driven by the anionic charge in the water—those polymer pieces remain charged. Breaking the polymer chain can be accomplished in different ways, including chemical, thermal and mechanical energy or combinations of all three.
Chemical energy: The conventional polymers used in chemical EOR are vulnerable to oxidization. The principal means of cleaning polymer from equipment is to use hydrogen peroxide or another oxidizer. Oxidation is able to break any remaining polymer in the water into smaller units, reducing the solution viscosity, which should improve the expected results of conventional separation equipment. But introducing oxygen to the equipment cleaning process promotes corrosion.
Thermal energy: Because polymers are susceptible to thermal degradation, a corresponding reduction in solution viscosity will occur. The susceptibility to thermal degradation will vary depending on the polymer and the water chemistry and often is not an effective means of achieving a viscosity reduction. Elevating the temperature can drive precipitation of polymers or polymer fragments with calcium, iron and other cations in the produced water. In many oil fields, when polymer waters with high total dissolved solids become heated, hard and tenacious coatings/deposits will form inside equipment downhole or on the surface. Removing these precipitates requires a substantial amount of mechanical force.
Mechanical energy: Once in solution, polymers also can be broken when exposed to physical shear forces like passing through a pump. Polymers are designed to resist mechanical shearing so that more polymer remains when pushed through the formation. The degradation caused by the formation varies, but in many fields only a 50% to 70% reduction in viscosity occurs. If the aggressive pumping downhole and through the formation only achieves a limited degradation, an excessive amount of mechanical energy will be required to destroy the remaining polymer. In any case, there are some operators that discourage destruction of the residual polymer because additional polymer has to be purchased to replace what has been destroyed.
Focus on the dispersed phase
Looking at the dispersed phase for answers would involve finding the means to disrupt the stabilizing mechanisms at the oil droplets/particles interface with the polymer water (continuous) phase. While there are currently few efforts being made in this area, there are technologies in various stages of development that “skip over” the continuous phase, which might work directly on interfacial tension.
Polymers
Regarding most of the past efforts to advance water process development, it was held that the nature of the polymers is a constant. Polymer chemistry is not treated as a variable. The insistence on using linear anionic homopolymers or copolymers ensures that water treatment capabilities will remain challenging, and precipitation of polymers with the sparingly soluble cations in produced water and corrosion and biological problems associated with polymers coating system surfaces will remain.
The inability to properly process produced water containing polymers often has caused operators to avoid using what is an effective EOR technique. There are plenty of opportunities for technological developments that would improve the overall conditions associated with polymer and ASP floods. But, short of inventing a new water treatment system, the results realized by altering existing equipment are likely to be disappointing.
Improving the control and efficiency of treating water used in polymer and ASP floods calls for two key developments: finding methods to directly destabilize the interface layer between the oil droplets/particles and the continuous phase without being encumbered by the conditions in the continuous phase and designing polymers that do not cling to steel surfaces as a result of overwhelming the chemical matrix with anionic charge. Research in these areas seems to hold the most promise for finding a polymer water that is readily treated for reinjection, other reuse or further processing and discharge.
Recommended Reading
Buffett: ‘No Interest’ in Occidental Takeover, Praises 'Hallelujah!' Shale
2024-02-27 - Berkshire Hathaway’s Warren Buffett added that the U.S. electric power situation is “ominous.”
Occidental Increases Annual Dividend by 22%
2024-02-11 - Occidental Petroleum Corp.’s newly declared dividend is at an annual rate of $0.88 per share, compared to the previous annual rate of $0.72 per share.
CEO: Magnolia Hunting Giddings Bolt-ons that ‘Pack a Punch’ in ‘24
2024-02-16 - Magnolia Oil & Gas plans to boost production volumes in the single digits this year, with the majority of the growth coming from the Giddings Field.
Why Endeavor Energy's Founder Sold His Company After Years of Rebuffing Offers
2024-02-13 - Autry Stephens', the 85-year-old wildcatter, decision to sell came after he was diagnosed with cancer, according to three people who discussed his health with him.
Sunoco’s $7B Acquisition of NuStar Evades Further FTC Scrutiny
2024-04-09 - The waiting period under the Hart-Scott-Rodino Antitrust Improvements Act for Sunoco’s pending acquisition of NuStar Energy has expired, bringing the deal one step closer to completion.




